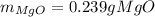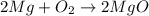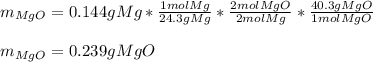Chemistry, 04.08.2020 14:01, denisebaslee15
· A 0.100g sample of Mg when combined with O2 yields 0.166g of Mgo, a
second Mg sample with a mass of 0.144g is also combined with O2. What
mass of MgO is produced from the second sample?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2


Do you know the correct answer?
· A 0.100g sample of Mg when combined with O2 yields 0.166g of Mgo, a
second Mg sample with a mass...
Questions in other subjects:

Business, 22.01.2021 18:30





Mathematics, 22.01.2021 18:30

English, 22.01.2021 18:30