
Chemistry, 01.08.2020 14:01, unknown1246
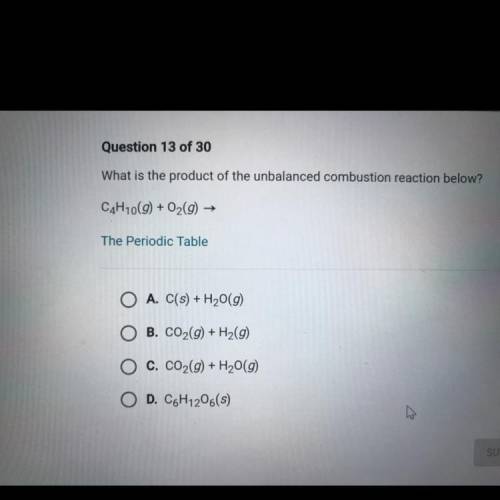
What is the product of the unbalanced combustion reaction below?
C4H10(g) + O2(g) →

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
Do you know the correct answer?
What is the product of the unbalanced combustion reaction below?
C4H10(g) + O2(g) →
...
C4H10(g) + O2(g) →
...
Questions in other subjects:


History, 27.05.2021 19:10



Mathematics, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10






