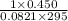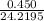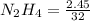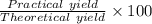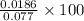Chemistry, 31.07.2020 20:01, gapaxton22
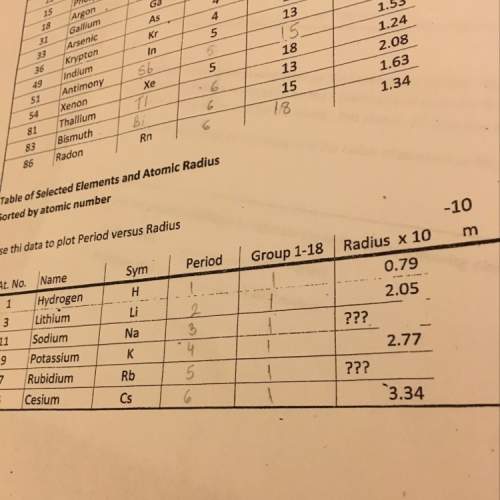
Hydrazine, N2H4 , reacts with oxygen to form nitrogen gas and water. N2H4(aq)+O2(g)⟶N2(g)+2H2O(l) If 2.45 g of N2H4 reacts with excess oxygen and produces 0.450 L of N2 , at 295 K and 1.00 atm, what is the percent yield of the reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
Do you know the correct answer?
Hydrazine, N2H4 , reacts with oxygen to form nitrogen gas and water. N2H4(aq)+O2(g)⟶N2(g)+2H2O(l) If...
Questions in other subjects:

History, 18.12.2019 23:31

Geography, 18.12.2019 23:31






Mathematics, 18.12.2019 23:31

Mathematics, 18.12.2019 23:31


 =
=