
Chemistry, 29.07.2020 16:01, hurtadocrv
Which of the following solutions would have the highest pH? Assume that they are all 0.10 M in acid at 25°C. The acid is followed by its Ka value.
a. HCHO2, 1.8 x 10-4
b. HF, 3.5 x 10-4
c. HClO2, 1.1 x 10-2
d. HCN, 4.9 x 10-10
e. HNO2, 4.6 x 10-4

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
Do you know the correct answer?
Which of the following solutions would have the highest pH? Assume that they are all 0.10 M in acid...
Questions in other subjects:

Mathematics, 06.07.2019 17:00






Mathematics, 06.07.2019 17:00




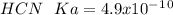
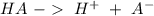
![Ka=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0714/8162/39962.png)
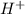 . Therefore, if we have a higher Ka value we will have a smaller pH (lets keep in mind that with a higher
. Therefore, if we have a higher Ka value we will have a smaller pH (lets keep in mind that with a higher 



