Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2


Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
Do you know the correct answer?
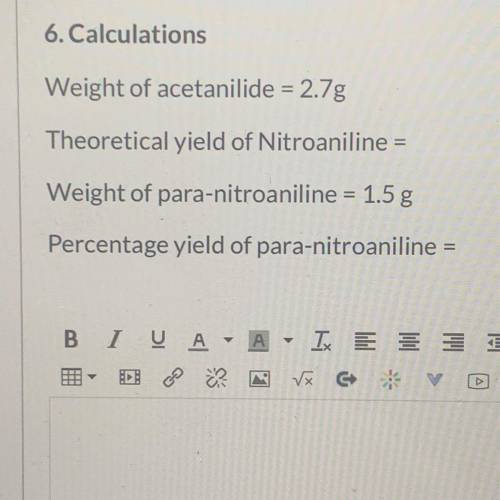
Calculations
Weight of acetanilide = 2.7g
Theoretical yield of Nitroaniline =
Weight of...
Theoretical yield of Nitroaniline =
Weight of...
Questions in other subjects:



Social Studies, 28.06.2019 19:30

Mathematics, 28.06.2019 19:30

History, 28.06.2019 19:30

Business, 28.06.2019 19:30

Social Studies, 28.06.2019 19:30


History, 28.06.2019 19:30

English, 28.06.2019 19:30