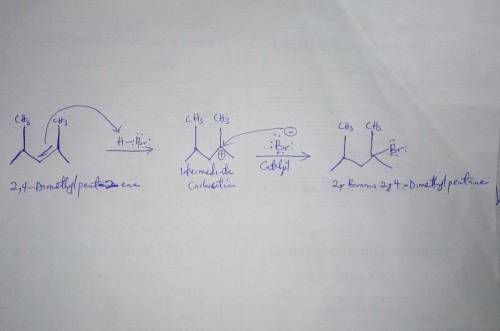
2,4-Dimethylpent-2-ene undergoes an electrophilic addition reaction in the presence of HBr to form 2-bromo-2,4-dimethylpentane. Complete the mechanism of this addition and draw the intermediates formed as the reaction proceeds. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Do you know the correct answer?
2,4-Dimethylpent-2-ene undergoes an electrophilic addition reaction in the presence of HBr to form 2...
Questions in other subjects:




Social Studies, 09.11.2020 17:00