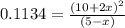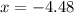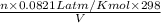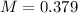Chemistry, 29.07.2020 04:01, leilaneedshelp3395
Consider the equilibrium system: N2O4 (g) = 2 NO2 (g) for which the Kp = 0.1134 at 25 C and deltaH rx is 58.03 kJ/mol. Assume that 1 mole of N2O4 and 2 moles of NO2 are introduced into a 5 L contains. What will be the equilibrium value of [N204]?
A) 0.358 M
B) 0.042 M
C) 0.0822 M
D) 0.928 M
E) 0.379 M

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
Do you know the correct answer?
Consider the equilibrium system: N2O4 (g) = 2 NO2 (g) for which the Kp = 0.1134 at 25 C and deltaH r...
Questions in other subjects:









English, 28.08.2019 20:10


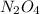 is 0.379 M
is 0.379 M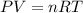
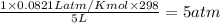
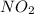 =
= 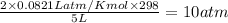
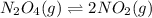
![K_p=\frac{[p_NO_2]^2}{[p_N_2O_4]}](/tpl/images/0714/6163/a9e41.png)