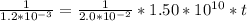Odine atoms will combine to form I2 in liquid hexane solvent with a rate constant of 1.5×1010L/mol⋅s. The reaction is second order in I . Since the reaction occurs so quickly, the only way to study the reaction is to create iodine atoms almost instantaneously, usually by photochemical decomposition of I2. Suppose a flash of light creates an initial [I] concentration of 2.00×10−2 M . How long will it take for 94% of the newly created iodine atoms to recombine to form I2? Express your answer using two significant figures.

Answers: 1
Other questions on the subject: Chemistry


Do you know the correct answer?
Odine atoms will combine to form I2 in liquid hexane solvent with a rate constant of 1.5×1010L/mol⋅s...
Questions in other subjects:



History, 03.12.2020 19:50

English, 03.12.2020 19:50


Mathematics, 03.12.2020 19:50


Mathematics, 03.12.2020 19:50



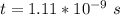
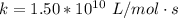
![[I] = 2.0*10^{-2} \ M](/tpl/images/0714/2862/c4b19.png)
![\frac{1 }{[I_r]} = \frac{1}{[I]} * k * t](/tpl/images/0714/2862/f99b3.png)
![[I_r]](/tpl/images/0714/2862/18947.png) is the concentration of the remaining iodine atom after the recombination which is mathematically evaluated as
is the concentration of the remaining iodine atom after the recombination which is mathematically evaluated as ![[I_r ] = [I_o ] *](/tpl/images/0714/2862/d1f12.png) [100% - 94%]
[100% - 94%]![[I_r ] = [I_o ] *0.06](/tpl/images/0714/2862/23b24.png)
![[I_r ] = 2.0 *10^{-2}*0.06](/tpl/images/0714/2862/d0235.png)
![[I_r ] = 1.2 *10^{-3}](/tpl/images/0714/2862/b5fbd.png)