
In each of the following groups of substances, pick the one that has the given property. Justify your answer. a) highest boiling point: HBr, Kr, Cl2 b) highest freezing point: H2O, NaCl, Hf c) lowest vapor pressure at 25C: Cl2, Br2, I2 d) lowest freezing point: N2, CO, CO2 e) lowest boiling point: CH4, CH3CH3, CH3CH2CH3 f) highest boiling point: HF, HCl, HBr Could someone help me understand fully how to do this?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
Do you know the correct answer?
In each of the following groups of substances, pick the one that has the given property. Justify you...
Questions in other subjects:


History, 10.05.2020 11:57

Computers and Technology, 10.05.2020 11:57





Mathematics, 10.05.2020 11:57



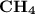 has the lowest molecular weight, thus the lowest freezing point.
has the lowest molecular weight, thus the lowest freezing point.



