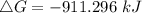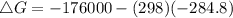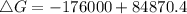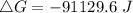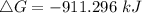Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
. Calculate ΔG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do...
Questions in other subjects:

Mathematics, 28.02.2020 20:48


Mathematics, 28.02.2020 20:48

Mathematics, 28.02.2020 20:48



History, 28.02.2020 20:48


Mathematics, 28.02.2020 20:48

Spanish, 28.02.2020 20:48