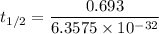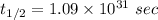Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
Do you know the correct answer?
The activation energy (E*) for 2N2O ---> 2N2 + O2 is 250 KJ. If the k for this reaction is 0.380/...
Questions in other subjects:




Mathematics, 27.04.2021 20:40


Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40


Mathematics, 27.04.2021 20:40


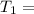 1001 K
1001 K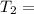 298 K
298 K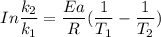
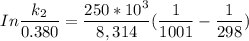
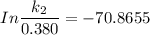

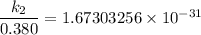
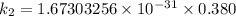
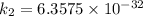 /M .sec
/M .sec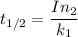
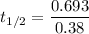
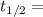 1.82368 secc
1.82368 secc