
Chemistry, 27.07.2020 01:01, ayoismeisjuam
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazoic acid is 2.20×10−5. Use the method of successive approximations in your calculations or the quadratic formula.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
Do you know the correct answer?
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazo...
Questions in other subjects:

Mathematics, 07.12.2021 18:10

Biology, 07.12.2021 18:10


Mathematics, 07.12.2021 18:10


Mathematics, 07.12.2021 18:10


Mathematics, 07.12.2021 18:10

Biology, 07.12.2021 18:10


![[H^+]=0.000285](/tpl/images/0713/5320/5d625.png)
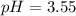
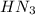 ). So:
). So:
![Ka=\frac{[H^+][N_3^-]}{[HN_3]}](/tpl/images/0713/5320/574a9.png)
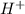 produced we will have 1 mol of
produced we will have 1 mol of 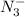 . So, we can use "X" for the unknown values and replace in the Ka equation:
. So, we can use "X" for the unknown values and replace in the Ka equation:![Ka=\frac{X*X}{[HN_3]}](/tpl/images/0713/5320/c6a9a.png)
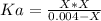
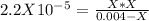
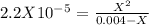
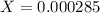
![pH=-Log[H^+]=-Log[0.000285]=3.55](/tpl/images/0713/5320/8cdec.png)





