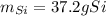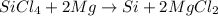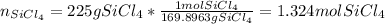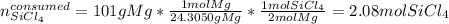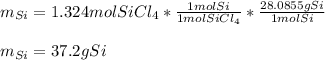Chemistry, 25.07.2020 04:01, genyjoannerubiera
What mass of Si, in grams, can be produced from the reaction below starting with 225 g SiCl4 and 101 g Mg? SiCl4 + Mg Si + MgCl2 Given: 1 mol SiCl4 = 169.8963 g SiCl4 1 mol Mg = 24.3050 g Mg 1 mol Si = 28.0855 g Si

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, KeiraDawn
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2



Do you know the correct answer?
What mass of Si, in grams, can be produced from the reaction below starting with 225 g SiCl4 and 101...
Questions in other subjects:


Mathematics, 13.03.2020 21:58

Mathematics, 13.03.2020 21:58

Mathematics, 13.03.2020 21:58