
Chemistry, 25.07.2020 03:01, jessica112776
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(1)= -174.5kJ/mol. I have no clue how to start this question and need help including the formulas so I know how to do it and some step by step commentary.


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, princessroyal
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1


Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
Do you know the correct answer?
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(...
Questions in other subjects:

Mathematics, 13.11.2020 01:30

Mathematics, 13.11.2020 01:30


Mathematics, 13.11.2020 01:30

History, 13.11.2020 01:30

Business, 13.11.2020 01:30

Mathematics, 13.11.2020 01:30

Arts, 13.11.2020 01:30

Mathematics, 13.11.2020 01:30

Social Studies, 13.11.2020 01:30


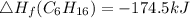
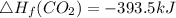
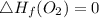
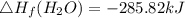
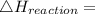 8 x - 393.5 - 8 x 285.82 + 174.5x 1
8 x - 393.5 - 8 x 285.82 + 174.5x 1 



