
Chemistry, 25.07.2020 03:01, mepuppy5000
A solution was made by mixing together 25.30 mL of liquid “A” and 36.20 mL of liquid “B” at a temperature of 53.80oC, within a closed container. The liquids have the following properties:
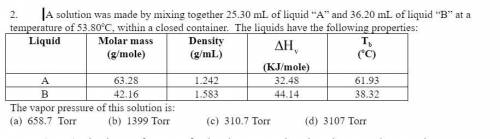

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 05:00, andrwisawesome0
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
Do you know the correct answer?
A solution was made by mixing together 25.30 mL of liquid “A” and 36.20 mL of liquid “B” at a temper...
Questions in other subjects:

Biology, 30.11.2021 23:50


Mathematics, 30.11.2021 23:50

Physics, 30.11.2021 23:50



Mathematics, 30.11.2021 23:50


Mathematics, 30.11.2021 23:50






