
Chemistry, 23.07.2020 14:01, phancharamachasm
Suppose you titrate 25.00 mL of 0.200 M KOBr with 0.200M H2SO4. The pH at half-equivalence point is 7.75 a). What is the initial pH of the 25.00mL of 0.200M KOBr mentioned above

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
Do you know the correct answer?
Suppose you titrate 25.00 mL of 0.200 M KOBr with 0.200M H2SO4. The pH at half-equivalence point is...
Questions in other subjects:




English, 29.07.2019 13:00

English, 29.07.2019 13:00


Computers and Technology, 29.07.2019 13:00

Mathematics, 29.07.2019 13:00

English, 29.07.2019 13:00

World Languages, 29.07.2019 13:00


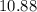 .
.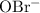 can act as a weak Bronsted-Lowry base:
can act as a weak Bronsted-Lowry base: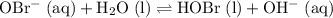 .
.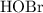 in this equation is
in this equation is 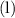 (meaning liquid) because
(meaning liquid) because 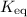 , isn't directly given. The idea is to find
, isn't directly given. The idea is to find 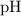 value at the half-equivalence point. Keep in mind that this system is at equilibrium all the time during the titration. If temperature stays the same, then the same
value at the half-equivalence point. Keep in mind that this system is at equilibrium all the time during the titration. If temperature stays the same, then the same ![\displaystyle K_\text{eq} = \frac{[\rm HOBr\; (l)]\cdot [\rm OH^{-}\; (aq)]}{[\rm OBr^{-}\; (aq)]}](/tpl/images/0711/7921/8c12d.png) .
.![\begin{aligned}& K_\text{eq} \\&= \frac{\left(\text{half-equivalence $[\rm HOBr\; (l)]$}\right)\cdot \left(\text{half-equivalence $[\rm OH^{-}\; (aq)]$}\right)}{\text{half-equivalence $[\rm OBr^{-}\; (l)]$}}\\ &=\text{half-equivalence $[\rm OH^{-}\; (aq)]$}\end{aligned}](/tpl/images/0711/7921/4e833.png) .
. 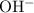 concentration at the half-equivalence point. Assume that
concentration at the half-equivalence point. Assume that 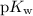 the self-ionization constant of water, is
the self-ionization constant of water, is 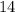 . The concentration of
. The concentration of ![\begin{aligned}& \text{half-equivalence $[\rm OH^{-}\; (aq)]$} \\ &= 10^{\rm pH - p\mathnormal{K}_\text{w}}\;\rm mol \cdot L^{-1} \\ &= 10^{7.75 - 14}\; \rm mol \cdot L^{-1}\\ &= 10^{-6.25}\; \rm mol \cdot L^{-1}\end{aligned}](/tpl/images/0711/7921/38e0b.png) .
.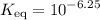 .
. is a soluble salt, all that
is a soluble salt, all that 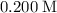 of
of 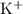 and
and ![\text{$[\rm OBr^{-}\; (aq)]$ before hydrolysis} = 0.200\; \rm M](/tpl/images/0711/7921/ea399.png) .
.![[\rm OH^{-}\; (aq)]](/tpl/images/0711/7921/bc9d8.png) be
be 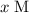 . Create a RICE table for this reversible reaction:
. Create a RICE table for this reversible reaction: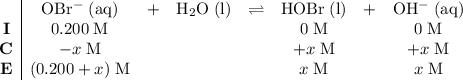 .
.![\displaystyle \frac{[\rm HOBr\; (l)]\cdot [\rm OH^{-}\; (aq)]}{[\rm OBr^{-}\; (aq)]} = \frac{x^2}{0.200 + x}](/tpl/images/0711/7921/e2d3c.png) .
. is much smaller than
is much smaller than 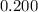 , such that the denominator is approximately the same as
, such that the denominator is approximately the same as ![\displaystyle \frac{[\rm HOBr\; (l)]\cdot [\rm OH^{-}\; (aq)]}{[\rm OBr^{-}\; (aq)]} = \frac{x^2}{0.200 + x} \approx \frac{x^2}{0.200}](/tpl/images/0711/7921/f87a1.png) .
.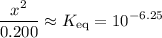 .
.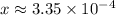 .
.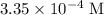 , which is the same as
, which is the same as 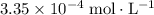 . Again, assume that
. Again, assume that 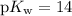 . Calculate the
. Calculate the ![\begin{aligned}\rm pH &= \rm p\mathnormal{K}_\text{w} + \log [\mathrm{OH^{-}}] \approx 10.88\end{aligned}](/tpl/images/0711/7921/8add0.png) .
. 



