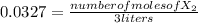Chemistry, 23.07.2020 21:01, trevorhenyan51
For the reaction system, H2(g) + X2(g) <--> 2HX(g), Kc = 24.4 at 300 K. A system made up from these components which is at equilibrium contains 0.150 moles of H2 and 0.600 moles of HX in a 3.00 liter container. Calculate the number of moles of X2(g) present at equilibrium.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
Do you know the correct answer?
For the reaction system, H2(g) + X2(g) <--> 2HX(g), Kc = 24.4 at 300 K. A system made up from...
Questions in other subjects:


History, 04.12.2019 05:31

History, 04.12.2019 05:31




Social Studies, 04.12.2019 05:31

Mathematics, 04.12.2019 05:31

Spanish, 04.12.2019 05:31


![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0711/9653/e45ae.png)
![Kc=\frac{[HX]^{2} }{[H_{2} ]*[X_{2} ]}](/tpl/images/0711/9653/32146.png)
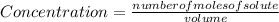 :
:![[H_{2} ]=\frac{0.150 moles}{3 liters}=0.05 \frac{moles}{liter}](/tpl/images/0711/9653/dc861.png)
![[HX]=\frac{0.600 moles}{3 liters}=0.2 \frac{moles}{liter}](/tpl/images/0711/9653/1aa32.png)
![24.4=\frac{0.2^{2} }{0.05*[X_{2} ]}](/tpl/images/0711/9653/346dd.png)
![[X_{2} ]=\frac{0.2^{2} }{0.05*24.4}](/tpl/images/0711/9653/ff5dd.png)
![[X_{2} ]=\frac{number of moles of X_{2} }{volume}](/tpl/images/0711/9653/ba7b5.png) , and the volume being 3 liters:
, and the volume being 3 liters: