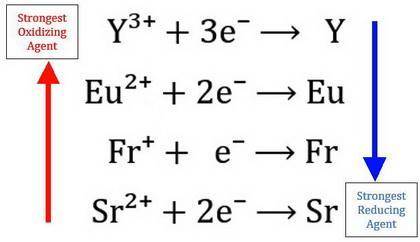Chemistry, 23.07.2020 09:01, Conpolice1309
. The four metals, Strontium(Sr), Francium (Fr), Yttrium (Y), and Europium (Eu), in separate experiments, are dipped in aqueous solutions of SrNO3, FrNO3, Y(NO3)3, and Eu(NO3)2. The following results are obtained: 1. Yttrium metal does not react with any of the solutions 2. Strontium metal reacts with all of the other metals solutions 3. Francium metal reacts in a solution of Eu(NO3)2 a) List the four oxidizing agents in order from strongest to weakest by creating a small reduction table. Explain your reasoning below b) List the four reducing agents in order from strongest to weakest

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
Do you know the correct answer?
. The four metals, Strontium(Sr), Francium (Fr), Yttrium (Y), and Europium (Eu), in separate experim...
Questions in other subjects:






Mathematics, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10