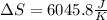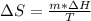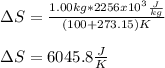Chemistry, 23.07.2020 01:01, janellesteele7498
Calculate the change in entropy when 1.00 kgkg of water at 100∘C∘C is vaporized and converted to steam at 100∘C∘C. Assume that the heat of vaporization of water is 2256×103J/kg2256×103J/kg. Express your answer in joules per kelvin.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
Do you know the correct answer?
Calculate the change in entropy when 1.00 kgkg of water at 100∘C∘C is vaporized and converted to ste...
Questions in other subjects:

Mathematics, 06.08.2021 21:50

Mathematics, 06.08.2021 21:50




Mathematics, 06.08.2021 21:50