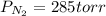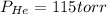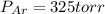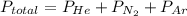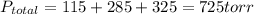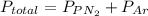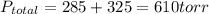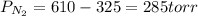A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pressures of 115, 285, and 325 torr, respectively. If all the He is removed from the mixture and the temperature does not change, what will be the partial pressure, in torr, of the N2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 15:30, vivianfling
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
Do you know the correct answer?
A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pre...
Questions in other subjects:


History, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00



Mathematics, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00