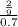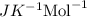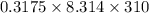Chemistry, 22.07.2020 23:01, MidnightAIY179
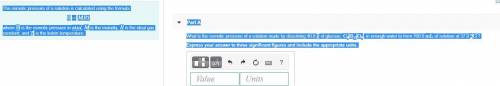
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pressure in atm, M is the molarity, R is the ideal gas constant, and T is the kelvin temperature. Part A What is the osmotic pressure of a solution made by dissolving 40.0 g of glucose, C6H12O6, in enough water to form 700.0 mL of solution at 37.0 ∘C ? Express your answer to three significant figures and include the appropriate units. nothing nothing

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
Do you know the correct answer?
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pres...
Questions in other subjects:



Chemistry, 22.04.2021 22:20

Computers and Technology, 22.04.2021 22:20


Chemistry, 22.04.2021 22:20

English, 22.04.2021 22:20

Mathematics, 22.04.2021 22:20


History, 22.04.2021 22:20


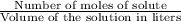
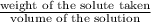
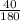
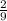 moles
moles