
Indicate the types of forces that are involved between the solute and solvent when forming a homogeneous solution between CH3CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH3.
a. dispersion forces
b. dipole-dipole forces
c. hydrogen bonding
d. ion-dipole forces
2. Indicate the types of forces that are involved between the solute and solvent when forming a homogeneous solution between LiNO3 and H2O.
a. dispersion forces
b. dipole-dipole forces
c. hydrogen bonding
d. ion-dipole forces

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2


Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
Indicate the types of forces that are involved between the solute and solvent when forming a homogen...
Questions in other subjects:

Mathematics, 06.11.2019 00:31

Social Studies, 06.11.2019 00:31



History, 06.11.2019 00:31




Health, 06.11.2019 00:31

Arts, 06.11.2019 00:31


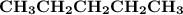 and
and 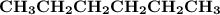
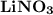 and
and 




