
1. A 10.0 g sample of propane, C3H8, was combusted in a constant-volume bomb
calorimeter. The total heat capacity of the bomb calorimeter and water was 8.0
kJ/°C. The molar heat of combustion of propane is -2 222 KJ/mol. If the starting
temperature of the water was 20 °C, what will be the final temperature of the
bomb calorimeter?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
Do you know the correct answer?
1. A 10.0 g sample of propane, C3H8, was combusted in a constant-volume bomb
calorimeter. The total...
Questions in other subjects:

Mathematics, 10.12.2020 04:40



Mathematics, 10.12.2020 04:40

English, 10.12.2020 04:40

Biology, 10.12.2020 04:40





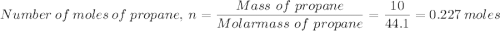 Which gives;
Which gives; in the calorimeter = Heat capacity × Temperature change
in the calorimeter = Heat capacity × Temperature change




