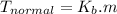Chemistry, 19.07.2020 14:01, umezinwachukwuebuka1
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as the solvent and the other has carbon tetrachloride as the solvent. Determine that molal concentration, m (or b), and boiling point, Tb.
Given
Ethanol
normal Boiling point: 78.4
Kb: 1.22
CCL4
normal boiling point: 76.8
Kb: 5.03

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, ksalinas7404
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 21.06.2019 22:00, juansantos7b
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2
Do you know the correct answer?
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as...
Questions in other subjects:



Mathematics, 09.10.2019 11:30



Social Studies, 09.10.2019 11:30




Computers and Technology, 09.10.2019 11:30


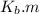
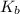 is the molal boiling point elevation constant;
is the molal boiling point elevation constant;