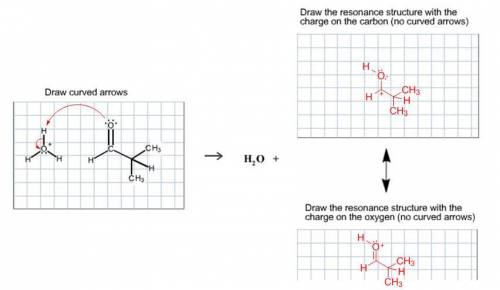
Chemistry, 19.07.2020 01:01, dontcareanyonemo
A proton transfer reaction can occur when a carbonyl compound is placed in an aqueous acidic solution. Water and a charged conjugate acid are produced. The resulting organic compound is resonance stabilized. Draw the curved arrows for the proton transfer and draw both resonance-stabilized organic products in the appropriate boxes. Be sure to include all lone pairs and nonzero formal charges. Do not draw the water product.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Do you know the correct answer?
A proton transfer reaction can occur when a carbonyl compound is placed in an aqueous acidic solutio...
Questions in other subjects:


Chemistry, 04.12.2019 22:31

English, 04.12.2019 22:31


Mathematics, 04.12.2019 22:31


Mathematics, 04.12.2019 22:31