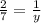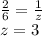Chemistry, 17.07.2020 14:01, NicoleParker
g If 1.00 mol of ethane gas and 5.00 mol of oxygen gas react, what is the limiting reactant and how many moles of water are produced from the reaction

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3


Do you know the correct answer?
g If 1.00 mol of ethane gas and 5.00 mol of oxygen gas react, what is the limiting reactant and how...
Questions in other subjects:

Business, 07.06.2021 05:30



Mathematics, 07.06.2021 05:30





Computers and Technology, 07.06.2021 05:30

Mathematics, 07.06.2021 05:30