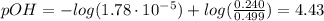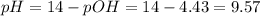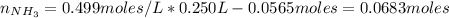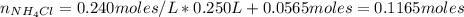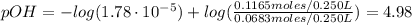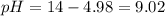Chemistry, 18.07.2020 01:01, DESIRE44030
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchloric acid are added to 250 mL of this buffer, what is the pH of the resulting solution? (Assume that the volume does not change upon adding perchloric acid.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
Do you know the correct answer?
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchlo...
Questions in other subjects:


Mathematics, 19.08.2019 22:50


History, 19.08.2019 22:50


Biology, 19.08.2019 22:50





![pOH = pKb + log(\frac{[NH_{4}Cl]}{[NH_{3}]})](/tpl/images/0708/9694/eb1d2.png)