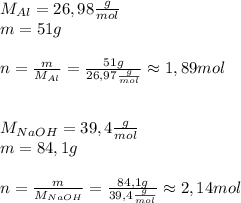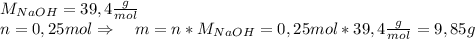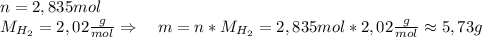Asolid piece of aluminum (51.0 g) was added to a solution of sodium hydroxide (84.1 g) in water, a balanced equation for this reaction is shown below:
2 naoh(aq)+ 2 al(s)+ 2 h2o → 2 naalo2(aq)+ 3 h2(g)
(a) which reagent is completely consumed by the reaction?
(b) after the reaction is completed, what is the mass of the reagent that remains? (c) what mass of hydrogen gas is produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3
Do you know the correct answer?
Asolid piece of aluminum (51.0 g) was added to a solution of sodium hydroxide (84.1 g) in water, a b...
Questions in other subjects:


Mathematics, 01.08.2021 01:10


Law, 01.08.2021 01:10

Mathematics, 01.08.2021 01:10





Mathematics, 01.08.2021 01:20