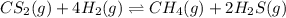The reaction system
CS2(g) + 4H2(g) CH4(g) + 2H2S(g)
is at equilibrium. Which of the followi...

Chemistry, 17.07.2020 19:01, haleynicole351ovewbg
The reaction system
CS2(g) + 4H2(g) CH4(g) + 2H2S(g)
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
a. As equilibrium is reestablished, the partial pressure of methane, CH4, decreases.
b. As equilibrium is reestablished, the partial pressure of carbon disulfide increases.
c. As equilibrium is reestablished, all the partial pressures will decrease.
d. As equilibrium is reestablished, the partial pressure of hydrogen sulfide decreases.
e. As equilibrium is reestablished, the partial pressure of hydrogen decreases.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


English, 26.08.2019 16:30


English, 26.08.2019 16:30

Chemistry, 26.08.2019 16:30

Mathematics, 26.08.2019 16:30


History, 26.08.2019 16:30


History, 26.08.2019 16:30