
Chemistry, 16.07.2020 17:01, payshencec21
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Calculate the equilibrium concentrations of reactants and product when 0.234 moles of H2 and 0.234 moles of I2 are introduced into a 1.00 L vessel at 698 K.
[H2] = M
[I2] = M
[HI] = M

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
Do you know the correct answer?
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Questions in other subjects:


Mathematics, 28.08.2019 10:10

History, 28.08.2019 10:10

Mathematics, 28.08.2019 10:10

Mathematics, 28.08.2019 10:10



Mathematics, 28.08.2019 10:10


History, 28.08.2019 10:10


![[I_2]=[H_2]=0.369M](/tpl/images/0707/8736/ed0a2.png)
![[HI]=0.0495M](/tpl/images/0707/8736/4fca5.png)
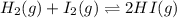
![Kc=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0707/8736/bf8a4.png)
 (considering the ICE procedure) is written as:
(considering the ICE procedure) is written as:![55.6=\frac{(2x)^2}{([I_2]_0-x)([H_2]_0-x)}](/tpl/images/0707/8736/e5efd.png)


![[I_2]=[H_2]=2*0.185M=0.369M](/tpl/images/0707/8736/ec590.png)
![[HI]=0.234-0.185=0.0495M](/tpl/images/0707/8736/318f7.png)




