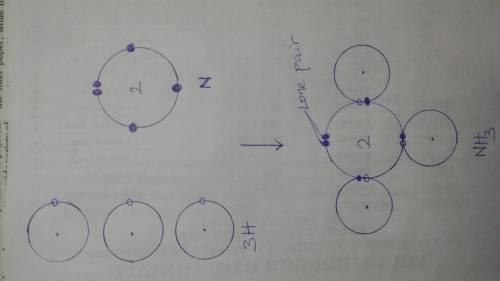
Chemistry, 16.07.2020 05:01, AleciaCassidy
Determine the number of lone pair electrons on each H in NH3. Z of N= 7, H=1 A. 0 B. 3 C. 1 D. 2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3
Do you know the correct answer?
Determine the number of lone pair electrons on each H in NH3. Z of N= 7, H=1 A. 0 B. 3 C. 1 D. 2
Questions in other subjects:

Biology, 23.09.2019 00:50



Social Studies, 23.09.2019 00:50

Social Studies, 23.09.2019 00:50

History, 23.09.2019 00:50


Mathematics, 23.09.2019 00:50

English, 23.09.2019 00:50

Mathematics, 23.09.2019 00:50