

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
Do you know the correct answer?
What is the pH of a buffer solution that contains 0.55 M methylamine, CH 3NH2, and 0.29 M methylammo...
Questions in other subjects:

Mathematics, 09.01.2022 14:00



Arts, 09.01.2022 14:00

Mathematics, 09.01.2022 14:00



English, 09.01.2022 14:00

Mathematics, 09.01.2022 14:00


![pH = pKa + log(\frac{[A^{-}]}{[HA]})](/tpl/images/0707/6504/7af75.png)
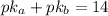
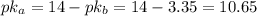
![pH = pKa + log(\frac{[A^{-}]}{[HA]}) = 10.65 + log(\frac{0.55}{0.29}) = 10.93](/tpl/images/0707/6504/f46ab.png)




