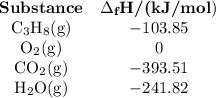Chemistry, 15.07.2020 20:01, mariakelley15
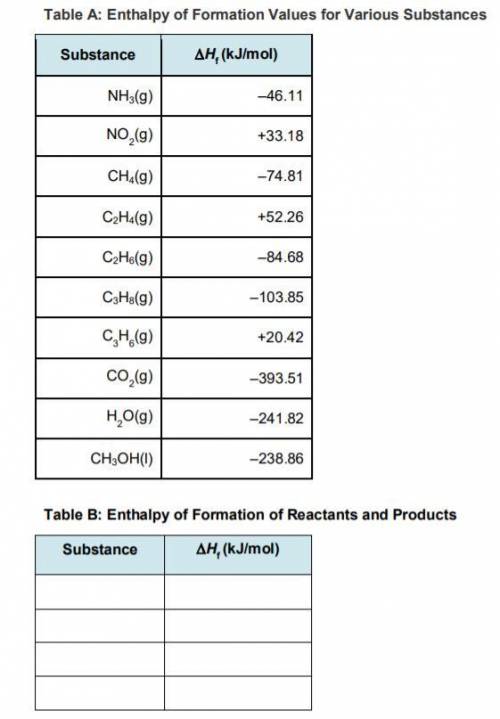
Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (∆Hf) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet. b) Determine the total enthalpy of the reactants and the total enthalpy of the products. Record these values in Table C of the Student Worksheet. c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic or exothermic.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, cristinaledford3696
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2


Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
Do you know the correct answer?
Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy values (...
Questions in other subjects:


History, 13.11.2020 18:20

English, 13.11.2020 18:20


Mathematics, 13.11.2020 18:20

Mathematics, 13.11.2020 18:20

Mathematics, 13.11.2020 18:20

Mathematics, 13.11.2020 18:20


Mathematics, 13.11.2020 18:20