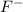Chemistry, 15.07.2020 04:01, Lydiaxqueen
When 100.0 mL of 0.40 M of HF and 100.0 mL of 0.40 M of NaOH are mixed, the resulting mixture is:a. a strong acid b. a strong base c. a weak acid d. a weak base e. a buffer

Answers: 2
Other questions on the subject: Chemistry




Do you know the correct answer?
When 100.0 mL of 0.40 M of HF and 100.0 mL of 0.40 M of NaOH are mixed, the resulting mixture is:a....
Questions in other subjects:







Social Studies, 24.12.2019 18:31

Spanish, 24.12.2019 18:31

Biology, 24.12.2019 18:31


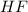 and
and 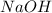 . So:
. So: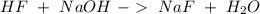
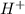

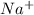 and the anion is
and the anion is