
Chemistry, 14.07.2020 02:01, sandyrose3012
A solution was prepared by dissolving 195.0 g of KCl in 215 g of water. Calculate the mole fraction of KCl. (The formula weight of KCl is 74.6 g/mol. The formula weight of water is 18.0 g/mol.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
A solution was prepared by dissolving 195.0 g of KCl in 215 g of water. Calculate the mole fraction...
Questions in other subjects:

Mathematics, 26.11.2020 19:00

Arts, 26.11.2020 19:00

Arts, 26.11.2020 19:00




English, 26.11.2020 19:10

Biology, 26.11.2020 19:10

Biology, 26.11.2020 19:10


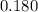 .
.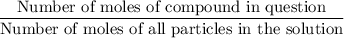 .
.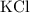 in this solution would be:
in this solution would be: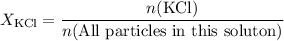 .
.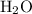 .) Hence:
.) Hence: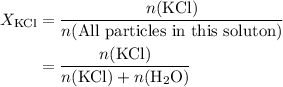 .
.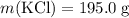 .Molar mass of
.Molar mass of 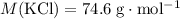 .Mass of
.Mass of 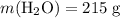 .Molar mass of
.Molar mass of 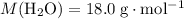 .
.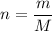 to find the number of moles of
to find the number of moles of  .
. .
. .
.




