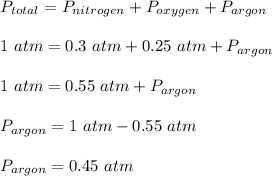Chemistry, 14.07.2020 23:01, smariedegray
The pressure of a 1-L nitrogen gas sample at 25 ⁰C is 0.30 atm. The pressure of a 1-L oxygen gas sample at the same temperature is 0.25 atm. The oxygen gas sample is added to the nitrogen container. Argon gas is added to the mixture until the total pressure of the 1-L container reaches 1.00 atm, and the temperature is adjusted to 25 ⁰C. According to Dalton’s Law of Partial Pressures, the contribution of each gas to the total pressure of the gas mixture is: Nitrogen Oxygen Argon

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1
Do you know the correct answer?
The pressure of a 1-L nitrogen gas sample at 25 ⁰C is 0.30 atm. The pressure of a 1-L oxygen gas sam...
Questions in other subjects:




Biology, 05.05.2020 16:02

Mathematics, 05.05.2020 16:02