
Chemistry, 14.07.2020 02:01, 20emmanuelg1030
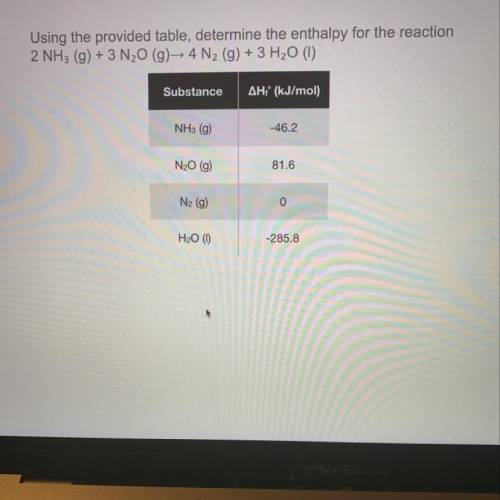
Using the provided table, determine the enthalpy for the reaction 2 NH3 (g) + 3 N20 (g) 4 N2 (g) + 3 H20 (1)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 21:50, BookandScienceNerd
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 23.06.2019 05:00, andrwisawesome0
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
Do you know the correct answer?
Using the provided table, determine the enthalpy for the reaction
2 NH3 (g) + 3 N20 (g) 4 N2 (g) +...
Questions in other subjects:

Mathematics, 03.11.2019 13:31

Social Studies, 03.11.2019 13:31


Mathematics, 03.11.2019 13:31

Mathematics, 03.11.2019 13:31

SAT, 03.11.2019 13:31

Mathematics, 03.11.2019 13:31


Mathematics, 03.11.2019 13:31

Biology, 03.11.2019 13:31






