

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
Do you know the correct answer?
A neutralization experiment using NaOH and HCl produced 716J. The limiting reactant was NaOH, which...
Questions in other subjects:




Mathematics, 12.08.2020 06:01

English, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01


History, 12.08.2020 06:01



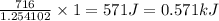 (1kJ=1000J)
(1kJ=1000J)




