
Chemistry, 15.07.2020 01:01, andersonrocksc
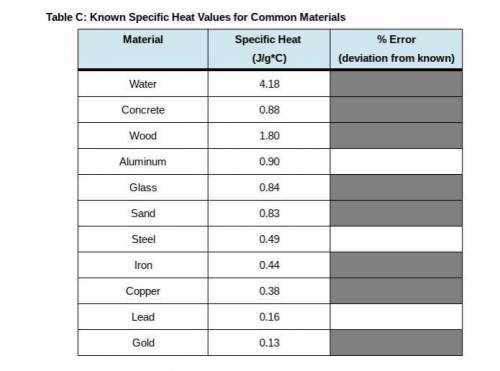
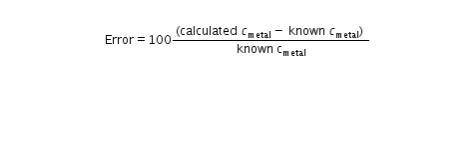
PLEASE I NEED HELP WITH THE BOXES ASAP !! in this last step, return to Step 10 in your Lab Guide to calculate the error between your calculated specific heat of each metal and the known values in Table C. Follow the directions given in your Lab Guide, using this formula: Error = 100 times StartFraction calculated c subscript metal minus known c subscript metal over known C subscript metal EndFraction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2


Do you know the correct answer?
PLEASE I NEED HELP WITH THE BOXES ASAP !! in this last step, return to Step 10 in your Lab Guide to...
Questions in other subjects:

Mathematics, 02.06.2021 16:30


English, 02.06.2021 16:30













