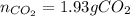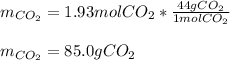Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
Do you know the correct answer?
Consider the combustion reaction for octane (C8H18), which is a primary component of gasoline.
2C8...
Questions in other subjects:


Social Studies, 16.09.2021 19:20



Mathematics, 16.09.2021 19:20

Business, 16.09.2021 19:20

English, 16.09.2021 19:20

Physics, 16.09.2021 19:20

Mathematics, 16.09.2021 19:20