
Chemistry, 14.07.2020 01:01, Prettygirlyaya
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40 at 346 K. (a) A pure sample of gaseous carbonyl bromide, COBr2(g), is introduced into a rigid flask at a temperature of 346 K so that its original pressure is 0.123 atm. Calculate the fraction of this starting material that is converted to products at equilibrium.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
Do you know the correct answer?
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40...
Questions in other subjects:






Health, 20.02.2020 02:11

History, 20.02.2020 02:11




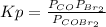 = 5.40
= 5.40



