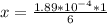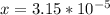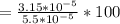Chemistry, 15.07.2020 01:01, cornpops4037
The average human body contains 5.00 L of blood with a Fe2+ concentration of 1.10×10−5 M . If a person ingests 9.00 mL of 21.0 mM NaCN, what percentage of iron(II) in the blood would be sequestered by the cyanide ion?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
Do you know the correct answer?
The average human body contains 5.00 L of blood with a Fe2+ concentration of 1.10×10−5 M . If a pers...
Questions in other subjects:

Mathematics, 24.08.2019 19:30

Chemistry, 24.08.2019 19:30

Mathematics, 24.08.2019 19:30


Health, 24.08.2019 19:30

Biology, 24.08.2019 19:30



History, 24.08.2019 19:30


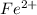
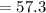 %
%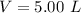
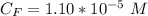
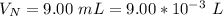
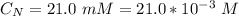
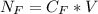
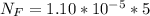
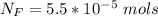
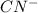 ingested is mathematically evaluated as
ingested is mathematically evaluated as 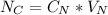
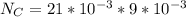

![Fe^{2+} + 6 CN^{-} \to [Fe(CN)_6]^{2-}](/tpl/images/0706/5120/f86b8.png)
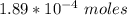 of
of