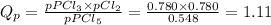Chemistry, 13.07.2020 21:01, ROBIOX5934
At T = 250 °C the reaction PCl5(g) PCl3(g) + Cl2(g) has an equilibrium constant in terms of pressures Kp = 2.15. (a) Suppose the initial partial pressure of PCl5 is 0.548 atm, and PPCl3 = PCl2 = 0.780 atm. Calculate the reaction quotient Qp and state whether the reaction proceeds to the right or to the left as equilibrium is approached

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
Do you know the correct answer?
At T = 250 °C the reaction PCl5(g) PCl3(g) + Cl2(g) has an equilibrium constant in terms of pressure...
Questions in other subjects:

Mathematics, 14.06.2020 09:57




Mathematics, 14.06.2020 09:57


Mathematics, 14.06.2020 09:57

Mathematics, 14.06.2020 09:57


Mathematics, 14.06.2020 09:57