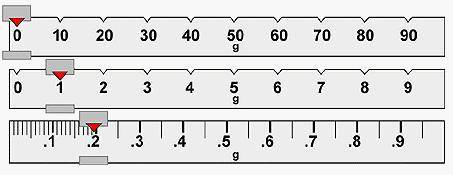
The unknown sample has a mass of: 101.20 g 1.019 g 11.2 g 1.195 g
...

Chemistry, 13.07.2020 21:01, coolquezzie
The unknown sample has a mass of: 101.20 g 1.019 g 11.2 g 1.195 g

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Mathematics, 01.09.2019 15:30




English, 01.09.2019 15:30

Geography, 01.09.2019 15:30

Social Studies, 01.09.2019 15:30







