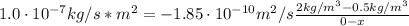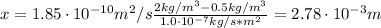A sheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900"C and is permitted to achieve a steady-state diffusion condition. The diffusion coefficient for nitrogen in steel at this temperature is 1.85 x 10^-10 m^2/s, and the diffusion flux is found to be 1.0 x 10^-7 kg/m^2 s. Also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 2 kg/m^3. How far into the sheet from this high-pressure side will the concentration be 0.5 kg/m3? Assume a linear concentration profile.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
Do you know the correct answer?
A sheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900"C and is permitted to ac...
Questions in other subjects:




Biology, 08.10.2019 06:00


History, 08.10.2019 06:00





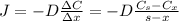
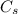 : is the nitrogen concentration in the surface of steel = 2 kg/m³
: is the nitrogen concentration in the surface of steel = 2 kg/m³ 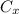 : is the nitrogen concentration in the point x = 0.5 kg/m³
: is the nitrogen concentration in the point x = 0.5 kg/m³