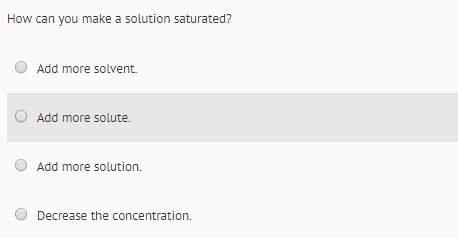
Chemistry, 14.07.2020 19:01, iamsecond235p318rq
Compounds X, C9H19Br, and Y, C9H19Cl, undergo base-promoted E2 elimination to give the same single alkene product, Z. Catalytic hydrogenation of Z affords 2,3,3-trimethylhexane. In water X readily reacts to give a substitution product, C9H19OH, together with Z. Y does not react with water. Propose structures for X and Y.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
Do you know the correct answer?
Compounds X, C9H19Br, and Y, C9H19Cl, undergo base-promoted E2 elimination to give the same single a...
Questions in other subjects:

Chemistry, 10.09.2019 23:10






Mathematics, 10.09.2019 23:10


History, 10.09.2019 23:10