Answers: 1
Other questions on the subject: Chemistry


Do you know the correct answer?
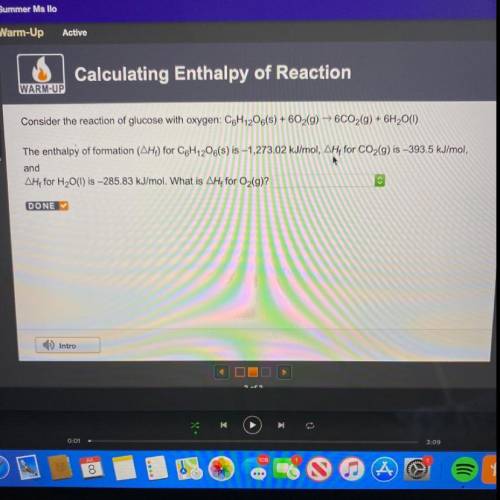
Consider the reaction of glucose with oxygen: C6H12O6(s) + 602(g) → 6CO2(g) + 6H2O(1)
The enthalpy...
Questions in other subjects:

Arts, 15.01.2021 21:50


Mathematics, 15.01.2021 21:50

English, 15.01.2021 21:50

Advanced Placement (AP), 15.01.2021 21:50

Computers and Technology, 15.01.2021 21:50


Mathematics, 15.01.2021 21:50