
Chemistry, 09.07.2020 01:01, southerntouch103
For the galvanic (voltaic) cell Fe(s) + Mn2+(aq) → Fe2+(aq) + Mn(s) (E°= 0.77 V at 25°C), what is [Fe2+] if [Mn2+] = 0.040 M and E = 0.78 V? Assume T is 298 K

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
For the galvanic (voltaic) cell Fe(s) + Mn2+(aq) → Fe2+(aq) + Mn(s) (E°= 0.77 V at 25°C), what is [F...
Questions in other subjects:

Mathematics, 12.04.2021 20:50

Mathematics, 12.04.2021 20:50

Mathematics, 12.04.2021 20:50


Mathematics, 12.04.2021 20:50

English, 12.04.2021 20:50

Biology, 12.04.2021 20:50

Chemistry, 12.04.2021 20:50



![\rm E_c_e_l_l\;=\;E_0\;-\;\dfrac{0.059}{n}\;\times\;log\;\dfrac{[Fe^+]}{[Mn^2^+]}](/tpl/images/0703/3772/e799c.png)
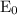 = initial voltage = 0.77 V
= initial voltage = 0.77 V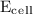 = cell voltage = 0.78 V
= cell voltage = 0.78 V![\rm \dfrac{0.059}{2}\;\times\;log\;\dfrac{[Fe^2^+]}{[0.040]}](/tpl/images/0703/3772/52542.png)
![\rm \times\;log\;\dfrac{[Fe^2^+]}{[0.040]}](/tpl/images/0703/3772/78e9d.png)
![\rm log\;\dfrac{[Fe^2^+]}{[0.040]}](/tpl/images/0703/3772/ac39f.png)
![\rm \dfrac{[Fe^2^+]}{0.040}](/tpl/images/0703/3772/f95e5.png)




