Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
Do you know the correct answer?
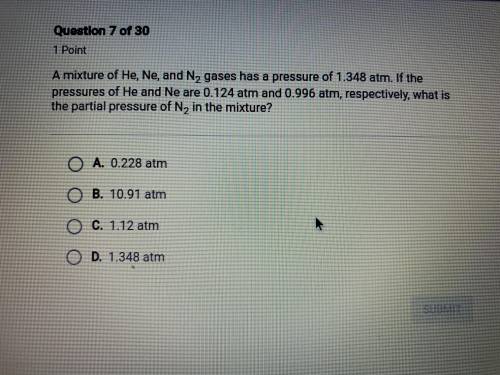
a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He and Ne are 0.1...
Questions in other subjects:


Mathematics, 16.07.2019 23:40

Geography, 16.07.2019 23:40



Mathematics, 16.07.2019 23:40

English, 16.07.2019 23:40



Mathematics, 16.07.2019 23:40