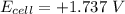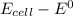Chemistry, 08.07.2020 02:01, gabbytumey
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s) + Zn(s) → 2Ag(s) + 2Cl– + Zn2+. If the zinc ion concentration is kept constant at 1 M, and the chlorine ion concentration is decreased from 1 M to 0.001 M, the cell voltage should:

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
Do you know the correct answer?
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s) + Zn(s) → 2Ag(s) + 2Cl–...
Questions in other subjects:

Geography, 26.02.2021 06:50

Biology, 26.02.2021 06:50

Mathematics, 26.02.2021 06:50


Mathematics, 26.02.2021 06:50

Mathematics, 26.02.2021 06:50

Mathematics, 26.02.2021 06:50


Mathematics, 26.02.2021 06:50


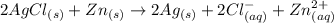
![E_{cell} = E^0- \dfrac{0,059}{n}log (\dfrac{[product]}{[reactant]})](/tpl/images/0702/8732/cb883.png)
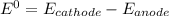
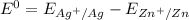
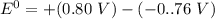
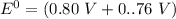
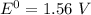
![E_{cell} = E^0- \dfrac{0.059}{n}log (\dfrac{[product]}{[reactant]})](/tpl/images/0702/8732/6f93c.png)
![E_{cell} = 1.56 - \dfrac{0.059}{2}log ({[Zn^{2+} ]}{[Cl^{2-}]})](/tpl/images/0702/8732/feb0e.png)
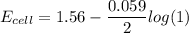
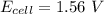
![E_{cell} = 1.56 - \dfrac{0.059}{2}log ({[1*0.001^2}]})](/tpl/images/0702/8732/27a68.png)
![E_{cell} = 1.56 - 0.0295 \ * \ log ({[1*10^{-6}}]})](/tpl/images/0702/8732/5785a.png)